
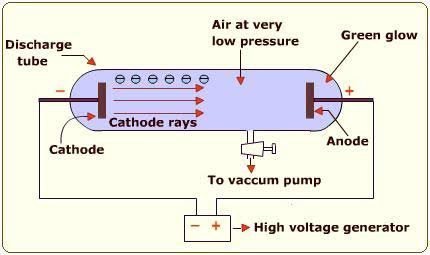
When a high voltage current was passed through the gas, shiny rays were emitted from the cathode which travels towards the anode as shown in the diagram below. These rays were given the name “cathode rays” as they originated from the cathode.
The cathode rays were studied in detail and their properties were determined, which are given
below:
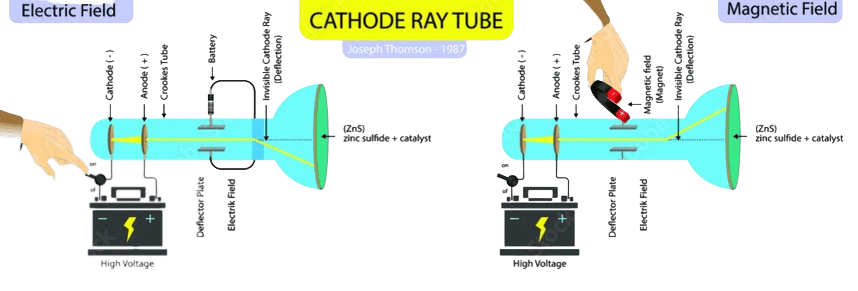
Different scientists tried different discharge tubes with different electrodes and different gases but they found the same results. Experiment shows that these particles could be produced by any kind of material. Cathode rays consist of particles that are known as ELECTRONS.

0 of 10 Questions completed
Questions:
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
When a high voltage current is passed through a discharge tube filled with gas, cathode rays are emitted from the.
Cathode rays travel in straight lines that are.
What property of cathode rays allows them to cast a sharp shadow of an opaque object?
Who discovered the charge-to-mass ratio (e/m) of cathode rays?
In an electric field, cathode rays are deflected towards the:
When cathode rays hit the walls of the discharge tube, what is produced?
Experimentally, it was found that cathode rays were emitted regardless of.
What did scientists conclude about the particles in cathode rays?
Cathode rays are:
The discovery of cathode rays led to the identification of particles known as: