
The atoms of an element that have the same number of protons but a different number of Neutrons are called Isotopes.
The naturally occurring isotopes of hydrogen are three, present in different abundances. The three isotopes of hydrogen are named
(i) Protium
(ii) Deuterium
(iii) Tritium
Each one of them has 1 proton and 1 electron, but the number of neutrons is different. A more accurate definition of an electron is “An electron is a subatomic particle which bears charge – 1.60 ×10–19 coulomb charge and – 1 elementary charge unit and has mass 9.1 × 10– 28 g.”
A more accurate definition of the proton is “A proton is a subatomic particle which bears charge + 1.60 × 10 –19 coulomb charge and + 1 elementary charge unit and has mass 1.67 × 10 – 24 g.”
A more accurate definition of the neutron is “A neutron is a subatomic particle which has a mass almost equal to that of a proton and has no charge.”

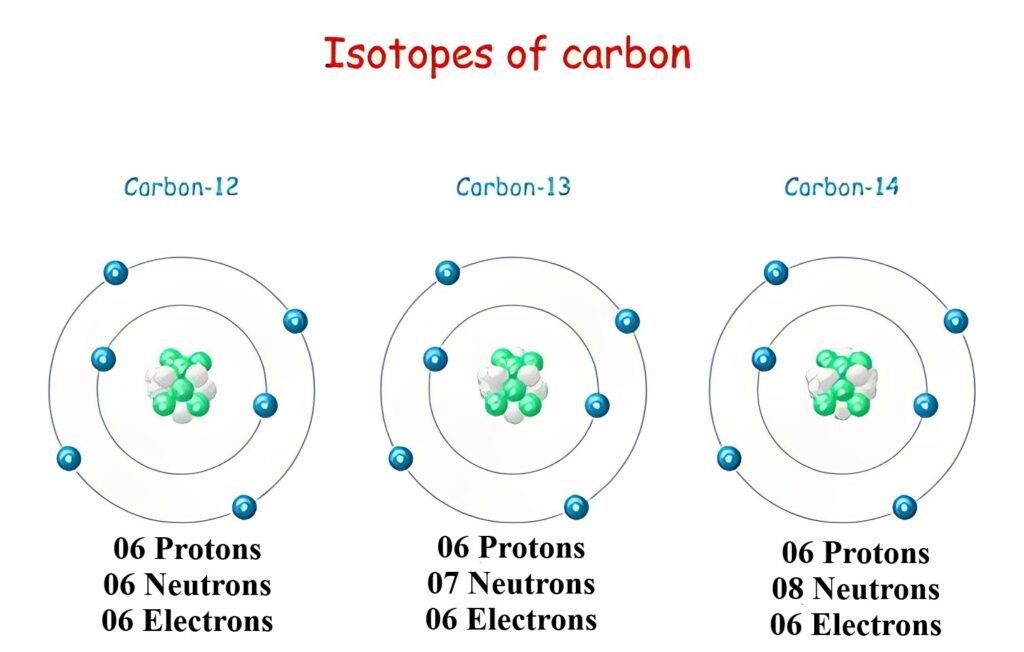
There are a total of three isotopes of carbon that exist, two of which are stable isotopes: 12C and 13C, and one is a radioactive isotope:14C.
The isotope 12C is present in nature with an abundance of 98.9%. On the other hand, both 13C and 14C are present in only 1.1% abundance.
All three isotopes of carbon have the same number of protons and electrons but differ in the number of neutrons.
There are two isotopes of chlorine found in nature i.e.17Cl35,17Cl 37 75%, and 25% respectively.
There are three isotopes of uranium all are radioactive i.e. 92U234,92U235, and 92Cl236 is found in nature nearly 99%.
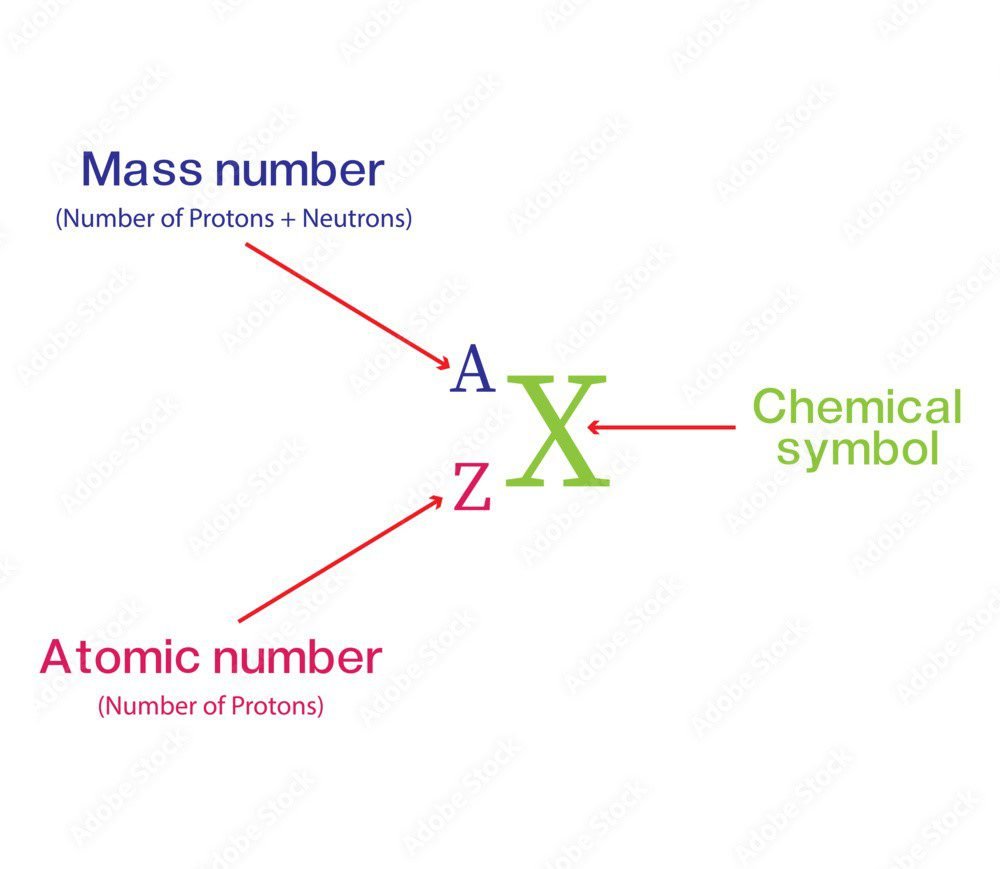
The number of protons present in the nucleus is called the Atomic number. It is denoted by the letter ‘Z’.
The mass number of an atom is the sum of the number of protons and neutrons in the nucleus of the atom. It is denoted by the letter ‘A’
A = p + n
The above equation is also called Nuclear equation.
The mass number is denoted by “A” and the atomic number is represented by “Z”, ‘X’ shown in the diagram is any element like C, N, O, and so on…
The atomic number represents the protons. The number of protons and electrons is equal to Z in an isolated or neutral atom. If we see any element like [ZXA = 6C12] so the subscript (base) represents Z and the superscript (power) represents A.
An energy level refers to the specific amount of energy that an electron can possess in an atom or molecule. These energy levels are sometimes referred to as electron shells.


0 of 10 Questions completed
Questions:
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Which subatomic particle determines the atomic number of an atom?
Which of the following isotopes of hydrogen is radioactive?
Which isotopes of carbon are stable?
What is the mass number of an atom?
Which isotopes of chlorine are naturally occurring?
Which isotopes of uranium are radioactive?
What does the atomic number represent in an atom?
What is an energy level in an atom?
Which sub-energy level is represented by the letter “d”?
How many subshells or sub-energy levels are there in an atom?