
Ionic bonds are formed by the complete transfer of electrons from one atom to another.
Ionic bonds are formed between atoms that have an electronegativity difference greater than about 1.9.
The bond formation between sodium and chlorine, a metal and a non-metal.
The electronegativity values of sodium and chlorine are 0.9 and 3.0 respectively. This tells us that sodium has a low ionization energy and a tendency to give electrons while chlorine has a tendency to take electrons. When those two atoms come together under suitable conditions, to complete their octets, sodium gives one electron to chlorine.
11Na : 1s2 , 2s2 , 2p6 , 3s1
17Cl : 1s2 , 2s2 , 2p6 , 3s2 , 3p5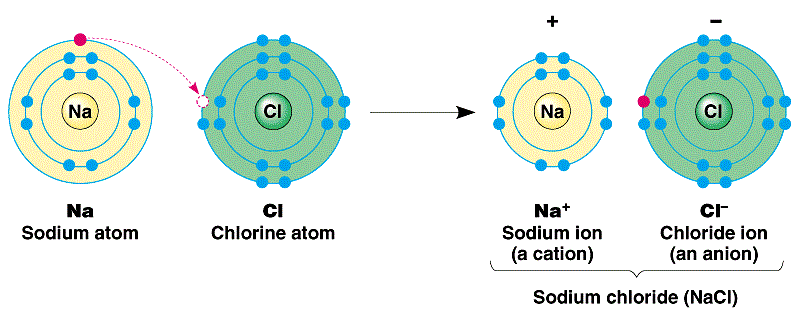
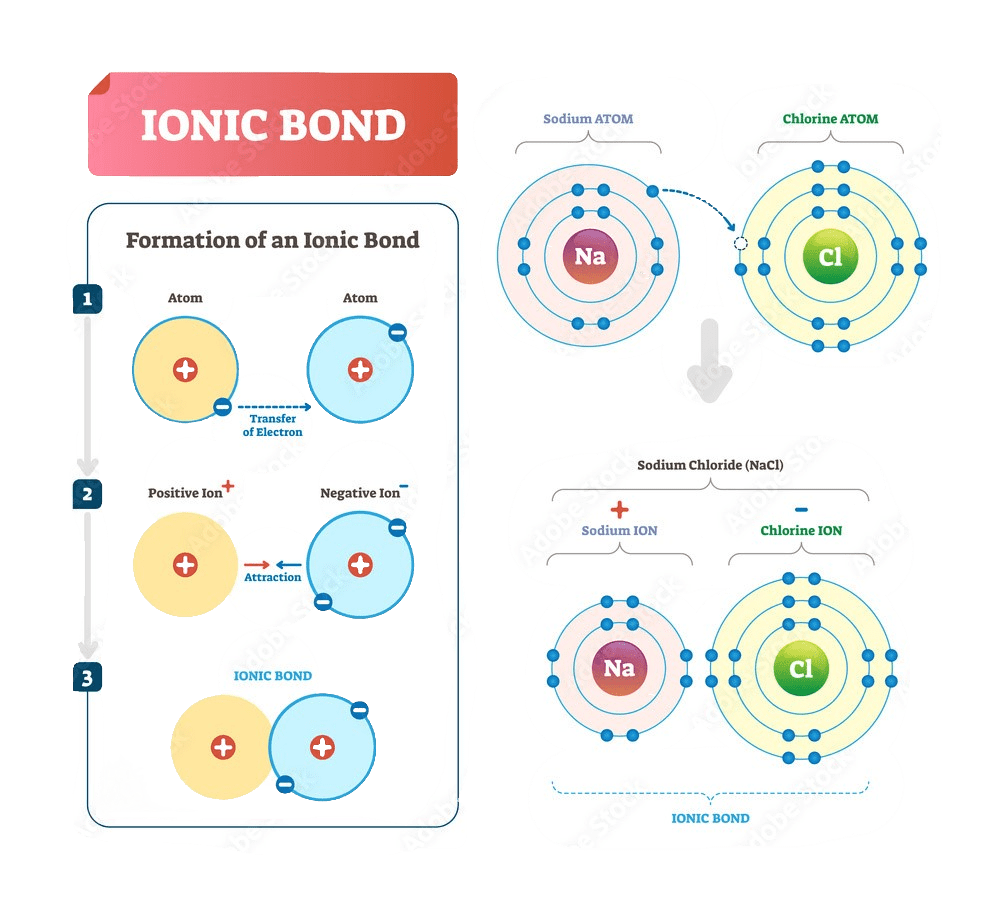
Sodium loses its valence electron and its electron configuration becomes identical to that of neon: 1s2 2s2 2p6. Likewise, the valence shell of chlorine becomes completely filled and its electron configuration resembles that of argon.


0 of 10 Questions completed
Questions:
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Ionic bonds are formed by the complete transfer of:
Ionic bonds are typically formed between atoms with an electronegativity difference greater than:
In the formation of an ionic bond between sodium and chlorine, sodium:
The electronegativity value of chlorine is:
The electron configuration of sodium after losing its valence electron is similar to that of:
The electron configuration of chlorine after gaining an electron is similar to that of:
Magnesium oxide is formed when magnesium:
The electron configuration of magnesium before bonding is:
The electron configuration of oxygen before bonding is:
In the formation of an ionic bond, which element typically loses electrons?