
“Enzymes are proteins that catalyze (i.e., speed up) biochemical reactions and are not changed during the reaction.”
During metabolism, chemicals are transformed from one form to another by enzymes. They act as biocatalysts and speed up and regulate metabolic activity.
Many enzymes are used commercially in industries. The most common industries are.
To get cellulose for paper making.
For making Bakery products and pizza.
For conversion of sugar into alcohol.
Used to remove different type of stains.
Enzymes are very sensitive to the environment in which they work. The activity of an enzyme is affected by any change in condition that alters its chemistry and shape. Some of the factors that can affect the rate of enzyme action are being discussed below.
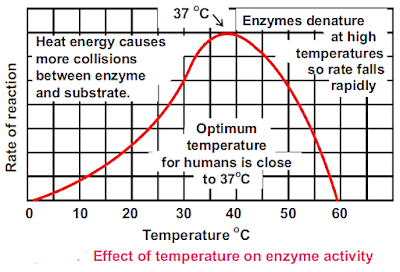
Increases in temperature speed up the rate of enzyme catalyzed reactions, but only to a point.
Every enzyme works at its maximum rate at a specific temperature called as the optimum temperature of that enzyme. When temperature rises to a certain limit, the heat adds in the activation energy and also provides kinetic energy and so reactions are accelerated.
When temperature is raised well above the optimum temperature, the heat energy increases the vibrations of atoms of enzymes and the globular structure of enzymes is lost. This is known as denaturation of enzymes.It results in a rapid decrease in the rate of enzyme action and it may be blocked completely.
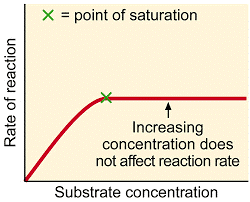
If there are enzymes molecules with vacant sites, an increase in substrate concentration will increase the rate of reaction.
If the enzyme’s concentration is kept constant and the amount of substrate is increased, a point is reached where any further increase in substrate does not increase the rate of reaction any more. When all the active sites of the enzymes are occupied at (high substrate concentration), any more substrate molecules do not find free active sites. This state is called saturation of active sites and does not increase the rate of reaction.
All enzymes work at their maximum rate at a narrow range of pH, called as theoptimum pH. A slight change (increase or decrease) in this pH causes retardation in enzyme activity or blocks it completely.
Every enzyme has its specific optimum pH values for example,
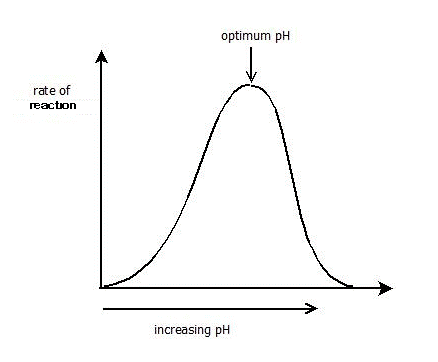
Change in pH can affect the ionization of the amino acids at the active sites.
Enzymes catalyze the reaction by attaching to substrate which ends to the product formation. Enzymes expose its active site to attract specific substrate, make enzyme substrate complex(ESC) after which the product is formed and enzyme is detached from it and used again for the same reaction.
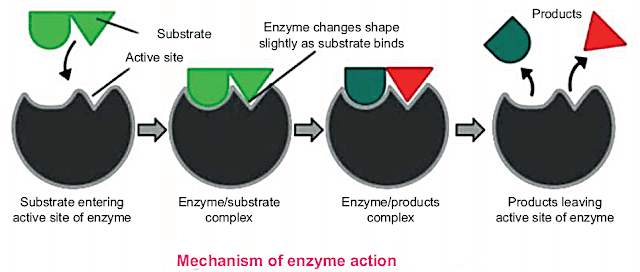
In order to explain the mechanism of enzyme action, a German chemist Emil Fischer, in 1894 proposed the lock and key model.

According to this model, both enzyme and The substrates possess specific shapes that fit exactly into one another. This model explains enzyme specificity.
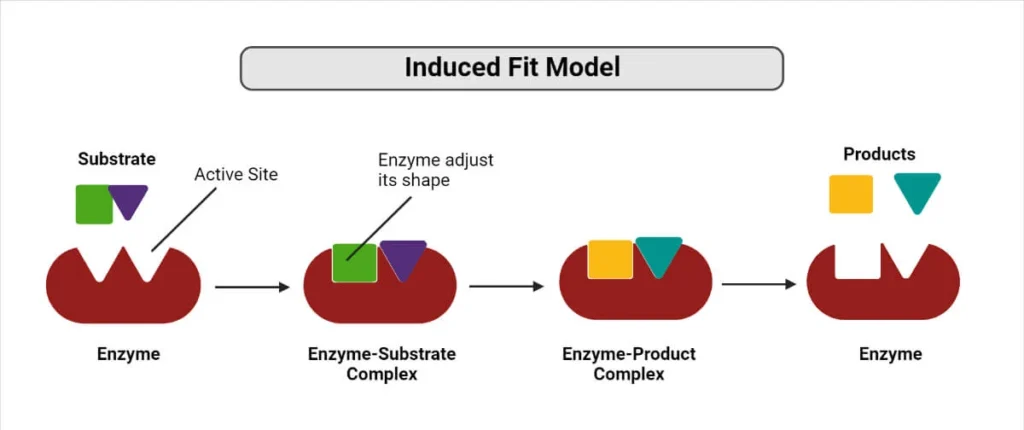
The induced fit model suggested by Daniel Koshland in 1958, explains that the active site continuously changes its shape until the substrate binds to it. It also said that active sites of enzymes are flexible (lock and key theory does not explain it.)
The chemical reaction requires particular conditions to carry down at proper rate, temperature and pressure. The condition of temperature and pressure inside a cell or organism are generally found not suitable for chemical reaction e.g., inside the human body normal temperature remains 37℃ and pressure is 120/80m.m of Hg. These conditions of temperature and pressure are not enough to perform any chemical reactions.
Now the body requires some facilitators.These facilitators help to perform biochemical reactions at low energy. It is clear now that each reaction requires some amount of minimum energy to initiate a reaction. This minimum amount of energy is called activation energy. If this amount is high, the difficulty will be the reaction of vice versa e.g., the activation energy needed to break a glucose molecule initially requires energy of 2 ATP molecules.
An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme’s activity can kill a pathogen.
| Activator | Inhibitor |
|---|---|
|
Function: Activators increase the activity of enzymes. They "turn on" the enzyme and enhance its catalytic function. |
Function: Inhibitors decrease the activity of enzymes. They "turn off" the enzyme and reduce its catalytic function. |
|
Action: Activators bind to the enzyme and induce a conformational change, making the enzyme more receptive to its substrate. |
Action: Inhibitors bind to the enzyme and block its active site, preventing the substrate from binding, and thus, inhibiting the enzyme's activity. |
|
Effect on Enzyme Activity: Activators increase the rate of enzyme-catalyzed reactions, leading to a faster conversion of substrate into product. |
Effect on Enzyme Activity: Inhibitors decrease the rate of enzyme-catalyzed reactions, slowing down the conversion of substrate into product. |
|
Regulation: Activators are involved in positive regulation, as they promote enzyme activity and are required for certain cellular processes to occur efficiently. |
Regulation: Inhibitors are involved in negative regulation, as they restrict enzyme activity and can serve as a control mechanism to prevent excessive product formation. |
|
Examples: An example of an activator is ATP (adenosine triphosphate), which activates various enzymes involved in energy-requiring processes. |
Examples: An example of an inhibitor is a competitive inhibitor, which competes with the substrate for the active site of the enzyme, reducing its activity. |
There are over 1000 known enzymes, each of which is involved in one specific chemical reaction. Enzymes are also substrate specific.
The enzyme protease (which breaks peptide bonds in proteins) will not work on starch (which is broken down by an enzyme amylose). Similarly, lipase enzyme acts only on lipids and digests them into fatty acids and glycerol.
The specificity of different enzymes is determined by the shapes of their active sites.
The active sites possess specific geometric shapes that fix with specific substrates.
The geometric shape of the active site of the given enzymes determines specificity for the substrate.
(Find out which substrate can exactly fit in the active site).

0 of 10 Questions completed
Questions:
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
What is the function of enzymes in biochemical reactions?
What is the role of enzymes in metabolism?
What are the reactants of enzymes called?
What is the active site of an enzyme?
How specific are enzymes in their action?
Which factors can affect the activity of enzymes?
What is the purpose of cofactors in enzyme functioning?
Which model explains the mechanism of enzyme action based on the complementary shapes of enzymes and substrates?
How many known enzymes are there?