
Chemical formula is a way of representing a substance using symbols and numbers. It tells us what elements make up the substance and how many of each element are present.
Compounds are made up of two or more different elements that are chemically bonded together. An element is a type of material that can’t be broken down into simpler substances by chemical means. So, when we combine two or more elements, we get a compound.
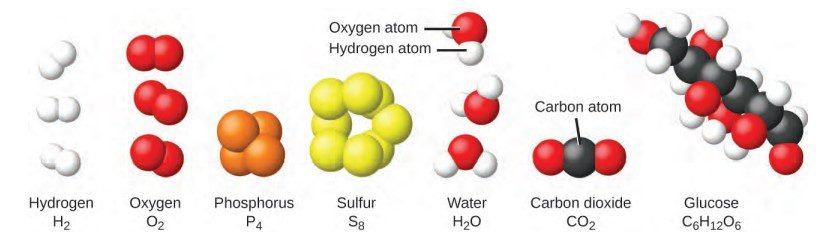
A mixture is when you combine two or more different pure substances physically.

When you mix things, they don’t change their chemical properties or combine to create something new. Instead, they just become physically combined. This means that you can usually still see and identify the different parts of the mixture. Mixtures may exist in either solid, liquid or gas.
In trail mix, you can still see the individual nuts and pieces of fruit.
A few of them are:
| Homogeneous Mixtures | Heterogeneous Mixtures |
|---|---|
| Uniform composition throughout the mixture. | Non-uniform composition throughout the mixture. |
| Particles are evenly distributed and cannot be easily distinguished. | Particles are unevenly distributed and can be easily distinguished. |
| Also known as solutions. | Also known as suspensions, colloids, or emulsions. |
| Examples include saltwater, air, and gasoline. | Examples include blood, salad dressing, and muddy water. |
| Can be separated by physical methods such as distillation and filtration. | Can be separated by physical methods such as centrifugation and sedimentation. |
| Properties are the same throughout the mixture. | Properties may vary throughout the mixture. |

| Property | Element | Compound | Mixture |
|---|---|---|---|
| Definition | A substance made up of only one type of atom | A substance made up of two or more different types of atoms chemically combined | A combination of two or more substances that are not chemically combined |
| Composition | Contains only one type of atom | Contains two or more types of atoms in a fixed ratio | Contains two or more substances in varying ratios |
| Properties | Has a unique set of physical and chemical properties | Has unique physical and chemical properties that are different from its constituent elements | May have properties that are similar to its constituent substances or new properties altogether |
| Separation | Can be broken down into simpler substances through chemical reactions | Can only be separated through chemical reactions | Can be separated through physical means such as filtration, distillation, and evaporation |
| Examples | Iron, Oxygen | Water, Carbon dioxide | Air, Saltwater |

0 of 10 Questions completed
Questions:
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Which of the following is an example of a homogeneous mixture?
A chemical formula represents:
What is the fundamental difference between a compound and a mixture?
Which of the following substances is a pure element?
How are elements represented in a chemical formula?
What happens to the properties of elements when they combine to form a compound?
A compound is made up of two or more different elements that are chemically bonded together.
Mixtures have a uniform composition throughout, with particles evenly distributed and indistinguishable.
Chemical formulas represent the physical properties of a substance rather than its chemical composition.
Elements can be broken down into simpler substances through chemical reactions.