
This law was given by J.Charles in 1787.
The volume of a given mass of a gas is directly proportional to the absolute temperature or Kelvin.
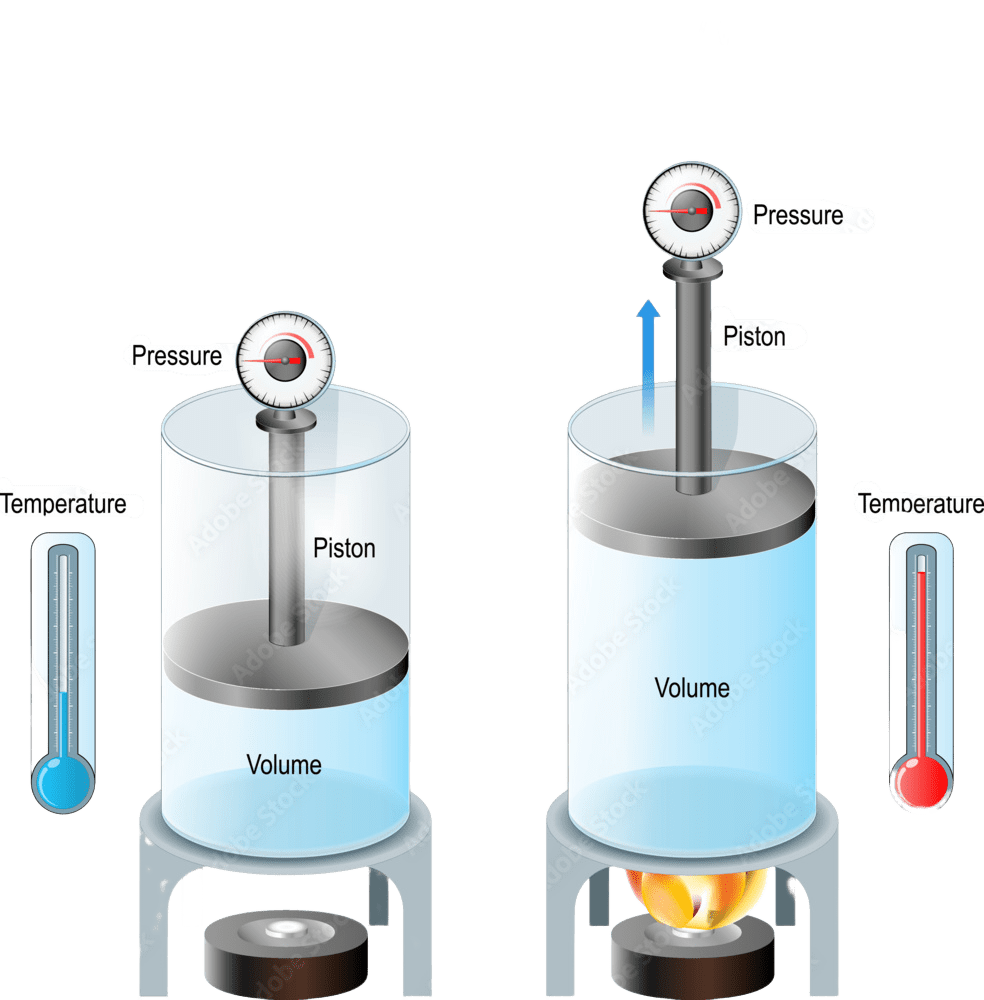
In other words, if the temperature of a gas is doubled at constant pressure, its volume also doubles, and if the temperature is halved, its volume is reduced to half. Mathematically, this law can be expressed as:
V/T = K
where V is the volume of the gas, T is its absolute temperature, and k is a constant of proportionality.
The equation can be rearranged as:
V1/T1 = V2/T2
where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.This equation is used to determine the volume of a gas at different temperatures or to calculate the temperature of a gas at different volumes. It is an essential tool in the study of thermodynamics and has many practical applications, including the design and operation of engines and refrigeration systems.
0 of 10 Questions completed
Questions:
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading…
You must sign in or sign up to start the quiz.
You must first complete the following:
0 of 10 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Charles’s Law describes the relationship between which two variables?
According to Charles’s Law, if the temperature of a gas is doubled while the pressure remains constant, what happens to its volume?
A gas occupies a volume of 2.0 L at a temperature of 300 K. If the temperature is increased to 600 K while the pressure remains constant, what will be the new volume of the gas?
Charles’s Law is applicable for which states of matter?
If the volume of a gas is halved while the temperature remains constant, what happens to its temperature according to Charles’s Law?
A gas at a temperature of 300 K occupies a volume of 2.0 L. If the temperature is increased to 600 K while the volume remains constant, what will be the new temperature of the gas?
Which graph represents the relationship between volume and temperature according to Charles’s Law?
Charles’s Law states that at constant pressure, the ratio of volume to temperature is constant. What is this constant called?
According to Charles’s Law, if the temperature of a gas is increased while the volume remains constant, what happens to its pressure?
Which of the following best describes Charles’s Law?